Which Statement Describes the Reactions in an Electrochemical Cell
BIt undergoes a spontaneous redox reaction. It permits the two solutions to mix completely.
1 equal to the total number of electrons gained.
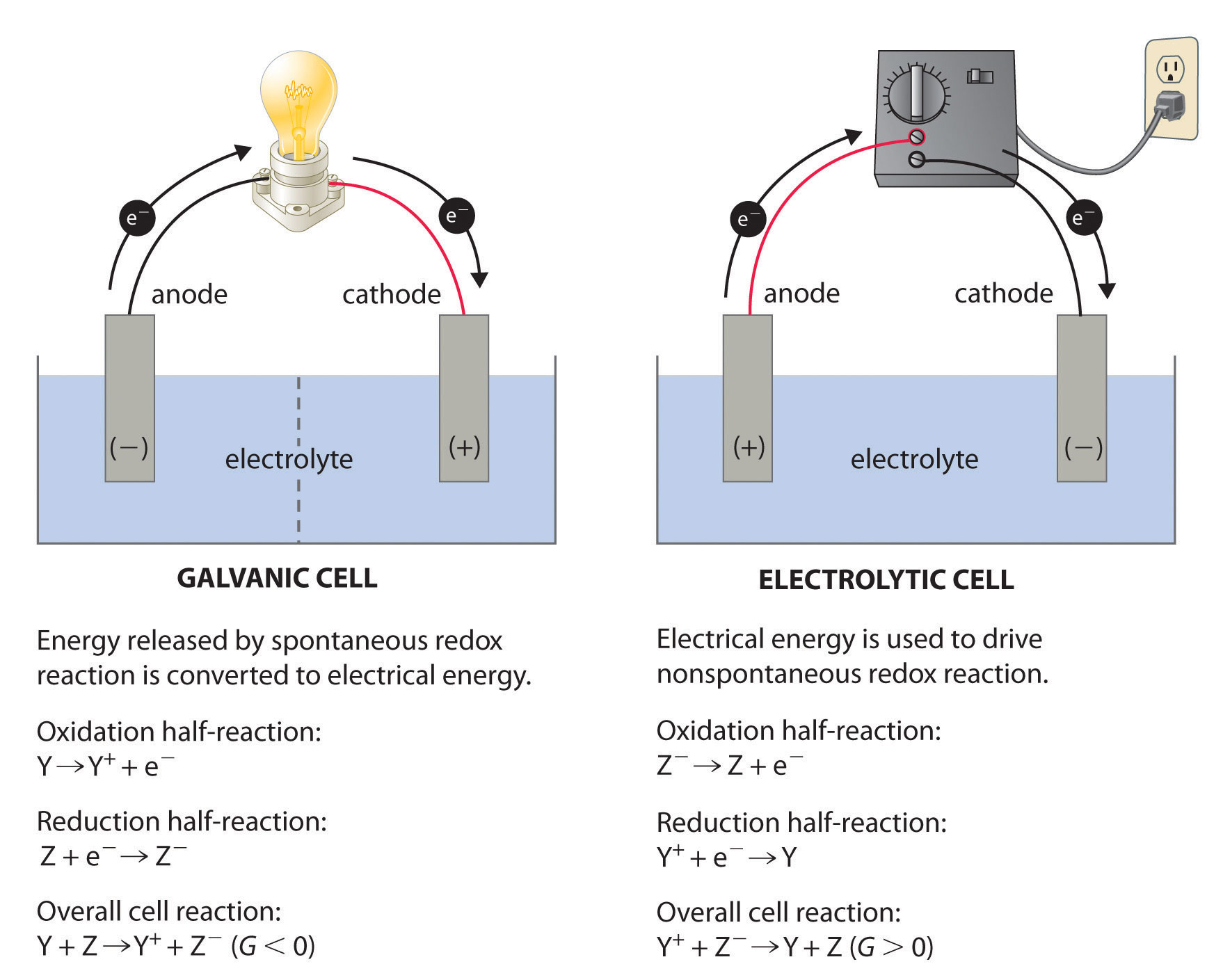
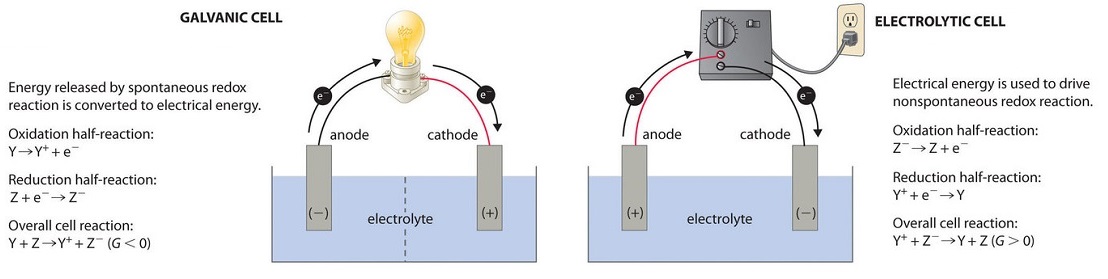
. DIt requires an external energy source. 8 still redox rxn. Electrochemical cells or batteries can be defined as devices capable of transforming chemical energy into electrical energy through spontaneous reactions of redox in which electron transfer occurs.
1 question Which statement correctly describes electrochemical cells. 3 electrical energy converted to chemical energy. 4 less than the total number of protons gained.
11Which statement describes one characteristic of an operating electrolytic cell. In an oxidation-reduction reaction the total number of electrons lost is. To calculate the Gibbs free energy change of the cell reaction.
4 polarities reversed- cathode - anode 5 still red cat an ox. To calculate the Equilibrium constant. To predict the spontaneity of the cell reaction.
22 Which statement describes where oxidation and reduction half-reactions occur in an. Which statement describes the reactions in an electrochemical cell. Which statement describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell.
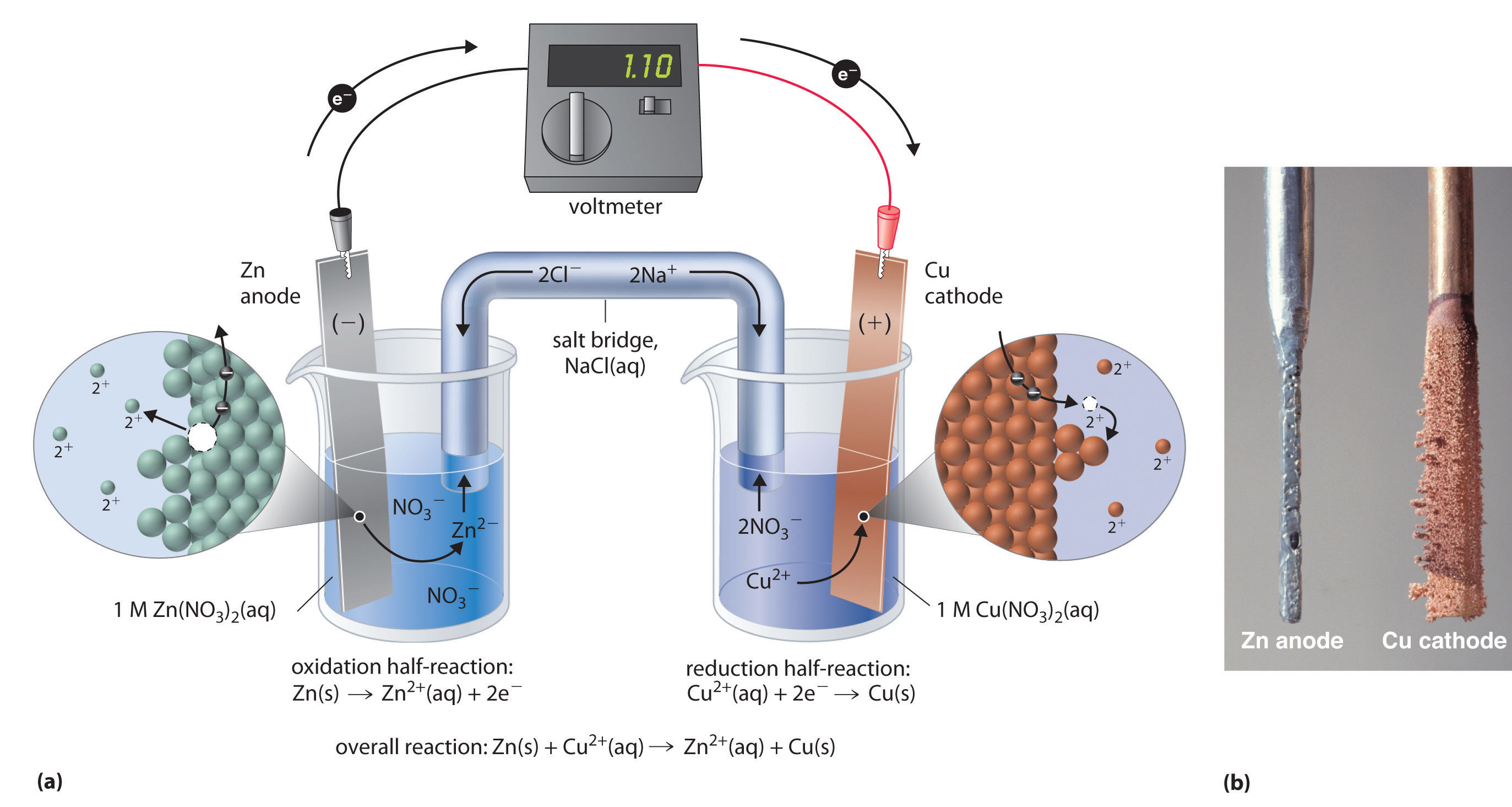
Redox it is a chemical reaction in which there is the occurrence of oxidation and reduction of atoms of substances chemical species present in the process. CHEM 1360 Test 5. The zinc electrode produces two electrons as it is oxidized Zn Zn2 2e Zn Zn 2 2 e which travel through the wire to the copper cathode.
Which of the following types of chemical reactions is necessary for electrochemical processes. ELECTROCHEMICAL CELLS Version 1 AIt produces electrical energy. In an electrochemical cell_____ Hard.
Given the reaction that occurs in an electrochemical cell. BYJUS Online learning Programs For K3 K10 K12 NEET. Zns CuSO4aq ZnSO4aq Cus During this reaction the oxidation number of Zn changes from 1 2 to 0 2 0 to -2 3.
Belectrochemical cells involve only reduction reactions. Aelectrochemical cells involve only oxidation reactions. 2 Oxidation occurs at the cathode and reduction occurs at the anode.
Given the balanced ionic equation representing the reaction in an operating voltaic cell. Sets with similar terms. CIt uses radioactive nuclides.
3 less than the total number of electrons gained. 6 e- still flow toward cathode. Which of these statements best describes a galvanic cell.
Zn Cu 2 Zn 2 Cu. 1 Oxidation occurs at the anode and reduction occurs at the cathode. 7 cathode still increases in mass.
Which of these statements b. Which statement describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell. When an electrically conducting device connects the electrodes the electrochemical reaction is.
2 equal to the total number of protons gained. Base your answer to the following question on the diagram below. 3 on a question Which statement describes where the oxidation and reduction half-reactions occur in an operating electrochemical cell.
Which statement best describes the flow of electrons in a voltaic or galvanic cell. Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids. Zns Cu²aq Zn²aq Cus The flow of electrons through the external circuit in this cell is from the a Cu anode to the Zn cathode b Cu cathode to the Zn anode c Zn anode to the Cu cathode d Zn cathode to the Cu anode.
2 redox rxn is non spontaneous. It prevents the reaction from occuring spontaneously it permits the migration of ions.

No comments for "Which Statement Describes the Reactions in an Electrochemical Cell"
Post a Comment